Лаборатория геологии техногенных процессов
Мaximovich N.G., Gorbunova K.A. Geochemical aspects of the geological medium changes in coal fields // Proceeding 6 Int. Congress Int. Ass. of Engineering Geology. A.A.Balkema.-Rotterdam,1990.-P.1457-1461. /0,4/
GEOCHEMICAL ASPECTS OF THE GEOLOGICAL MEDIUM CHANGES IN COALFIELDS CHANGEMENTS GEOCHIMIQUES D'ENVIRONNEMENT DANS LES GISEMENTS DE HOUILLE
N. G. Maximovich, K. A. Gorbunova
ABSTRACT: The character of the technogenic and geochemical processes in coal-extraction areas is determined by the origin and structure of coal-bearing formations and by their transformation in hypergenesis and technogenesis zone. fly the example of Kizel Goal Basin in the Urals it is shown that in sulphur containing coal-bearing strata there develops a sulphuric-acid process effecting all the components of the geological medium and engineering constructions.
RESUME: Le charactere des processus technogeniques et geochimiques dans les domaines d'extraction de carbon est determine par 1' origine et la structure de formations portant le carbon et leur transformation dans le zone de hypergenesis et technogenesis. On a montre sur 1' example des gisements de houille Kizel a 1' Oural que les stratoms portant le sulphur se develope un processus d' acide sulphurique qui influence sur tous les composants d' environnement geologique et constructions techniques.
I. Introduction
World coal resources, according to various international organizations, are estimated at 8 to 16 billion tons. The world annual output of coal makes up about 2 milliard tons, hereat a great amount of solid, liquid and gaseous waste being extracted from the interior part of the Earth.
In the USSR about 700 mines supply of the order of 500 million tons of coal a year accompanied by escape of 6 milliard cubic meters of methane, over 3 milliard cubic meters of carbon dioxide, 12.5 thousand tons of dust, about 1.4 milliard cubic meters of pit water and 110.8 million tons of rock /Iruni, 1979/.
Goal extraction and processing are accompanied with soil "alienation" and pollution of the environment. Under surface conditions the substances being extracted and the products of their processing are chemically unstable. There arise technogenetic and geochemical systems /Voronkevich, I980/ whose contrast with the natural ones causes an intensive substance migration, growth of irreversible physico-chemical processes considerably changing all the geological medium components and having a negative effect on engineering constructions.
2. Geochemical Features of Goal-Bearing Strata
Coal-bearing formations have a rhythmic structure. Goal seams are interlaid with sandstones, argillic rocks, more rarely with limestones. The coal and the containing rock composition includes carbon, hydrogen, oxygen, nitrogen /mainly in the organic substance/, silicon, aluminium, iron, calcium, magnium, potassium, sodium (mainly in the mineral part), sulphur and phosphorus (taking an intermediate position) (Clair et al., 1987).
One of the geochemically active components is sulphur. Its concentration in the USSR coals averages 1.5%, reaching in some deposits and seams 12% and even 20%. The sulphur is in sulphide, organic, sulphate and element forms.
At various stages of the coal-bearing formations forming-up and transformation (sedimentogenesis, diagenesis, catagenesis and hypergenesis) there change thermodynamic, redox, acidic-and-alkaline and biochemical conditions which determine the migrating capability of the elements and the forms of their existence. At the diagenesis stage under the effect of sulphate-reducing bacteria the sulphate sulphur is reduced to the element form and hydrogen sulphide with the consequent binding in the composition of hard-migrating sulphides and organic compounds. In the hypergenesis zone with the ground water oxygen present a biochemical oxydation of sulphides occurs. A sulphuric-acid process is developing.
3. Substance Migration while Coal Mining
The geochemical processes in the hypergenesis zone become active in connection with coal mining covering all the geological medium components (rocks, underground and surface waters, underground and surface atmosphere, biosphere). There appears a new type of technogenetic substance migration connected with technological operations of the rock extraction, its transportation and processing, pumping out of pit waters and gas (Fig. 1).
The geological medium is most greatly effected by: 1)pit waters, 2)coal extraction and preparation solid waste being stockpiled.
4. Geochemical Changes of Geological Medium
4.1. Pit Waters Effect
Coal mining is connected with pumping out of pit and drain waters. In Kizel Coal Basin,Ural region, due to intensive disturbance of the coal-bearing strata, great mining depths (up to 1 km), presence of karsted carbonate rocks in the overlaying sediments the mine inflows make up 80 to 300 m3/h and in the karst influence zone up to 2.500 m3 (Mironov, Polyakov, 1987). The pit waters chemical composition mainly depends on the hydrodynamic conditions, the sulphur, carbonates and trace elements content in the coal-bearing formation. With the sulphur content in coal of more than 4% as a result of sulphides oxidation the water acquires the pH value of 2 to 3 and a sulphate composition. In Kizel Coal Basin the fracture-and-karstic waters of the carbonate rocks possessing a high oxidation potential, neutral medium at pH 7.3 to 7.5, hydrocarbonate-calcium composition and mineralization of 0.06 to 1.5 g/l, interact in mines with the sulphur-rich coal-bearing rocks and change into sulphate ferric-aluminium sodium-calcium waters with mineralization of 2.5-19 to 35 g/l. The pit waters compared to the natural ones are enriched with microelements: lead, copper, zinc, nickel, cobalt and others. The basin annually disposes into the rivers over 100 million cubic meters of pit waters (Bankovskaya, Maximovich, 1987). One of the rivers in the natural conditions has a mineralization of 53 to 250 mg/l and a hydro-carbonate-calcium composition at pH 6.5 to 7.8.In the zone of coal mines effect the river water mineralization increases to 5 g/l at pH 2.3 to 3.1 with sulphate ion dominating (up to 3 g/l).In the oxidizing medium the iron oxides precipitate from the water and settle along the banks and on the bottom deposits in the form of reddish illinition.
4.2. Effect of Coal Extraction Solid Waste
With each thousand tons of mine extracted coal there are brought out onto the surface average 100 to 115 cubic meters of rock (Tyutyunova, 1987). In 1986 the total area of spoil heaps on the USSR territory made up 10,000 ha. They occupy considerable areas in Kizel Coal Basin. The coal extraction has been taking place there since 1797. The coal-bearing formation of the Visean stage of the Lower carboniferous series is made up of sandstones, aleurolites and argillites with coal interlayers. The upper part of the section is represented with limestones. The coal is highly sulphuric. It is composed of (average for the basin,%): pure coal 85, clay 9, pyrite 4, other admixtures 2. In some spots the pyrite content increases to 9% (Yeryomin, 1980).
The spoil heaps consist of argillite, sandstone and limestone fragments with coal inclusions. Therein a specific physico-chemical situation due to "joint presence" of a carbonic substance and pyrite. The rock transportation onto the surface from the lack-of-oxygen zone and dumping off the rock pressure make more active physical weathering, oxidation, solution, hydrolysis, hydrotation, metaso matosis and others.
A characteristic reaction is pyrite oxidation with a formation of sulphates and sulphuric acid which is an oxidizer. It decomposes silicates and alumosilicates. In acid medium at pH 2 to 3 many cationogenetic elements become mobile. Solutions get enriched with ferrum, aluminium, magnium, sodium and potassium. Oxidation reactions are accompanied with heat emission and self-ignition of spoil heaps. At burning sites temperatures of 1000°C and more there appear processes close to endogenetic by character. Burning of spoil heaps continues on for several years. The burning products pollute the atmosphere and deposit on the surface.
Weathering, roasting and remelt, pneumatolytic and fumarole phenomena form in spoil heaps a complex of natural technogenic minerals. Among the burnt spoil heaps of Chelyabinsk Coal Basin more than 40 types are described (Chesnokov et al., 1987).
In the mine spoil heaps of Kizel Coal Basin, by means of X-ray structural analysis and electron microscopy (Fig. 2) there have been determined the following minerals: virgins (sulphur),carbides (moissonite). sulphides (pyrite, marcasite), oxides and hydroxides (quartz, corundum, hematite, magnetite, limonite and others), carbonates (calcite, aragonite, siderite), sulphates (jarosite, alonite, gypsum, barite, melanterite and others), silicates (caoline, millite, sericite, illite, chlorite, feld- , spar and others). Both among the natural and the technogenic minerals there are present ones containing sulphur: pyrite, marcasite, alounite, gypsum and others. The sulphur sulphide content in different aged spoil heaps ranges from 3 to 8%. The rock reaction is strongly acidic at aqueous suspension pH 1.9 to 2.6 and in spoil heaps aged 50-3.2 (Nikiforova, Solntseva, 1986). In the waters filtrated through the spoil heaps and flowing down their surface the sulphate ion content comes up to 50 g/1 at pH I to 3 and mineralization of up to 70 g/l. In the composition of the surface runoff from the mine territory there were also found suspended substances, aluminium, ferrum, nickel, copper, lead, cobalt. The interaction with technogenic waters changes the ground properties.
5. The Technogenic Grounds Geochemical Effect on Engineering Constructions
The USSR accounts about 2.1 thousand spoil heaps of coal mines polluting the environment. To create an ecological balance recultivation and liquidation of spoil heaps through using them as mineral products or construction material is done. Use of more spoil in construction is only possible provided their composition is carefully studied and their content and forms of occurance of geochemically active components to be revealed as well as dynamics of the geochernical formation processes of aggressive media. This is confirmed by practical use of mine spoil rocks mixed with, natural ground in setting up a construction site for an industry in Kizel Basin.
The embanking technogenic grounds included 4-0 to 90% sulphur-containing mine spoil heaps. There in a local aquifer was formed whose level lay higher than the foot of the construction foundation. Within the observation period of 1934 through 1990 the water mineralization in some points reached 7 g/l with the sulphate ion content of 4.5 g/l. As the geochemical studies have shown, the water samples contain thiobacills which increase the oxidation intensity of sulphur compounds. The water changed from weakly aggressive into strongly aggressive to concrete
6. Aspects of Geochemical Processes Control
Coal-bearing deposits are characterized by reductional conditions of the formation. In the extraction process they change into oxydative ones which causes development of a sulphuric acid process in sulphur-containing coal-bearing formations. The technogenic and geochemical processes are accompanied by pollution of the atmosphere, surface and underground waters. The sulphuric acid process changes the ground filtration properties, forms acid sulphide media aggressive to concrete and metal construction. It contributes to development of landslides, karst and other hazardous geological processes.
A negative effect of the coal mining on the geological medium can be reduced by creating man-made geochemical barriers-alkaline, sulphate, oxygen, adsorbtional and biochemical ones by use of natural substances (oxygen of the air), rocks (Limestones), various industrial waste (for instance, ash slag). As experimental works have shown, a comparatively simple technological introduction of such barriers on sites is possible.
Literature
Iruni A.A. Environment Protection in Underground Coal Mining. Review. M., 1979, 47 p. Bankovskaya V.M. , Maximovich N.G. Protection of Rivers in the Kama Basin from Waste Waters Pollution from Coal Industry. All--Union Conference Abstracts. Tbilisi, 1987, p. 7-3
Voronkevich S.D. On Technogenic and Geochernical Systems in Engineering Geology. "Engineering Geology", 1930, No. 5, p.3-13-
Yeryomin I.V. Changes and Petrographic Features of Coals in Oxidation in Natural Conditions, M., 1980, 84 p. Clair V.R. et al. Metallogenesis and Geochemistry of Coal--Bearing and 3par-Containing Strata in USSR. M., Geochemistry of Elements, "Nauka", 1987, 238 p.
Mironov K.V., Polyakov V.F. Kizel Coal Basin. Mining Encyclopaedia, 1937, p.12, No.3,M.
Nikiforova E.M., Solntseva N.P. Technogenic Sulphur Flows in Humid Landscapes of Coal Mining Areas. M., 1936. No. 3, p.52--59- Moscow State University, 3er. 5
Tyutyunova F.I. Hydrochemistry of Technogenesis. M., "Nauka", 1987, 335 p.
Chesnokov B.V. Mineralogy of Burnt Spoil Heaps in Chelyabinsk Coal Basin. Sverdlovsk, 1987, 70 p.
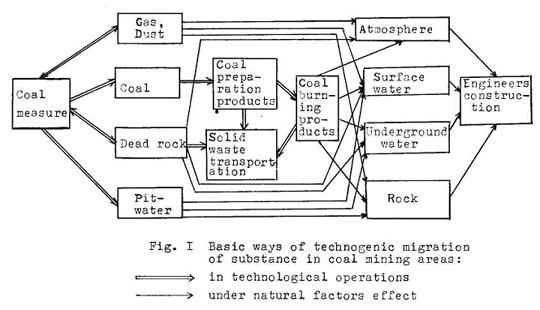 
Fig. 2 Electron micropicture of minerals from Kizel Basin spoil heaps:
a) jarosite, b) melanterite

|
|
|

