Лаборатория геологии техногенных процессов
Sergeev V.I., Shimko T.G., Kuleshova M.L., Maximovich N.G. Ground water protection against pollution by heavy metals at waste disposal sites// Wat.Sci.Tech,1996-Vol.34,-N7-8.-P.383-387.
GROUNDWATER PROTECTION AGAINST POLLUTION BY HEAVY METALS AT WASTE DISPOSAL SITES
V. I. Sergeev, T. G. Shimko, M. L. Kuleshova and N. G. Maximovich
ABSTRACT
The usefulness of subsoil layer as geochemical barrier against migration of heavy metals from waste disposal sites is assessed. Field and laboratory studies and theoretical calculation are reported that enable the forecasting of pollutant migration through different subsoils and the length of time that such soils will function on geochemical barriers. Copyright © 1996 IAWQ. Published by Elsevier Science Ltd.
KEYWORDS
Groundwater; subsoils; pollutant migration; heavy metals; waste-disposal sites.
INTRODUCTION
Groundwater is an important ingredient of the environment. The processes of groundwater pollution have reached impressive extent, especially in regions with mining and processing industries. Heavy metals are very common pollutants of groundwaters in these areas. This article presents an assessment of the subsoil layer as of a natural geochemical barrier on heavy metals migration pathways.
Sorption properties of clay subsoils with respect to heavy metals
Investigations have been carried out with clay samples different in mineralogy and granulometry, with heavy metals Cu, Zn, Cd, Co, Ni, Mn, Pb salts solutions, as well as with liquid phase of the feed pulp sampled at dressing mills. A sorption capacity determination was performed under static and dynamic conditions.
While determining the sorption capacity in statics (Nw) of primary importance is the correct choice of:
- subsoil-metal solution ratio;
- initial concentration of the element - sorbate in solution;
- optimal time of interaction between the subsoil and solution.
The results have shown that the maximum sorption capacity of the sample (Njr) for a given heavy metal has been obtained in two versions of experiments: by repeated replacement of the solution of the same concentration, being in contact with the same sample, or by determining the sorption isotherm by routine methods. In the latter case the sample is being treated by solutions with varying metals concentrations.
Isotherms for 21 variants of slayey subsoils of different origin were obtained in the experiments for copper, zinc, cadmium, cobalt, nickel and manganese (sulfates) and lead (nitrate); the results enabled us to determine Nst. These data are shown in Table 1 for some subsoils and monomineral clays. Granulometric and mineralogical compositions and the cation exchange capacity have been determined for all the samples.
The analysis of the results made it possible to reveal regularities in the sorption cpacity features, as well as agents exerting their effects on sorption processes.
1. In accordance with their sorption intensity the metals under study may be arranged in the following sequence: Pb > Cu > Zn > Cd, Co > Ni > Mn.
2. Sorption capacity for metals strongly depends on the carbonate content in samples and their buffering capacity by pH.
3. More elevated sorption capacity is associated with samples where montmorillonite and hydromica are dominant.
Nst, values, obtained under static conditions are adequate for potential sorption capacity, since the solution is interacting with non-disturbed material, so that each particle comes in contact with the solution. Such ideal conditions for reactions account for the approximate character of sorption capacity computation, based on determining N,,, values; nevertheless, they may be applied at the preliminary stage when rough assessment of the aeration zone is required for a site where wastes are stored.
Table 1. Sorption capacities Nst of subsoils for heavy metals (statics) 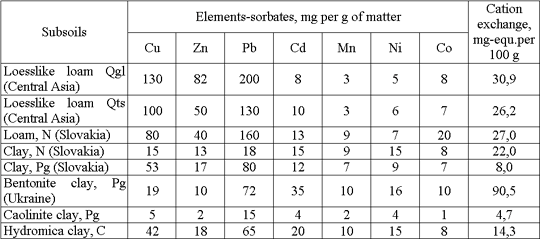
For evaluating the contribution of non-disturbed subsoil structure to the sorption capacity, experiments have been carried out with undisturbed samples in dynamic regime. The resulting curves in coordinates of relative concentration C of the volume V of the solution percolated through the sample are obtained. They serve to determine the sorption capacity under dynamic conditions (Nd).
The influence of percolation rate on the sorption process should be taken into account when designing the experiments, since the mass exchange for the majority of mineral particle surfaces proceeds in the diffusion sphere, and quick migration of solutes through active voids may create conditions for incomplete realization of the subsoil sorption capacity. The purpose of the experiments is to create a resulting breakthrough curve (Fig. 1).
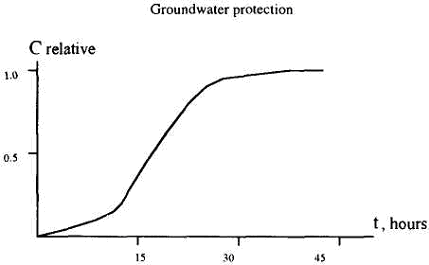 Figure 1. Resulting breakthrough curve Cd sorption by loesslike loam (solution CdSO4 initial concentration 440 mg/1 by Cd).
Determination of loesslike loam sorption capacity for copper, cadmium and manganese in dynamic conditions permitted us to assess the importance of the subsoil structure in the sorption process. The difference between Nst and Nd being 2-4.5 times, should be introduced into assessment of control functions of subsoils in the of undation of waste storage sites.
Choice of an adequate mathematical model describing migration process and determining migration parameters
Resulting curves for samples with undistrubed structure serve to quantify not only the sorption capacity, but also, the migration. They enable us to start forecasting changes of heavy metal concentration in subsoil in time.
Investigation shows the possibility of applying two models for natural subsoil or for artificial clay screen: block-heterogeneous and hydrodispersional in case of linear kinetics of sorption.
The model of block-heterogeneous medium with clustered opacity comprises a system of weakly permeable aggregates, regularly penetrated by water conducting canals. The model takes into account micro heterogeneity of the medium and presumes the linear kinetics of the sorption process development; it is presented by the following set of equations:
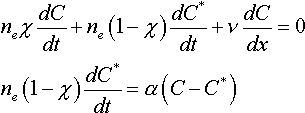
where n e - effective porosity; 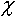 - relative porosity of canals; C and C*- matter concentration in canals and blocks, respectively; 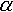 - mass exchange coefficient.
Analysis of the solution for the block-heterogeneous model has shown the appearance of the zone with lower concentrations adjacent to the front of the pollutant sprawl; it originates due to sorption kinetics despite the absence of hydrodispersive effect. Similar solutions of one-dimensional tasks of mass transport obtained by some authors indicate the presence of symptotic features in the solution, if the migration is prolonged, close to the solution of microdisperison equation, when w>10Kd/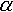 , where Kd = N/C, and where n and D are substituted by effective coefficients ne and De. , where Kd = N/C, and where n and D are substituted by effective coefficients ne and De.
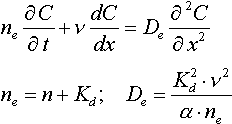 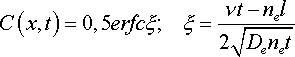
where n e - effective porosity; D e - effective disperstion coefficient; v - filtration rate; t - time of solution movement; l - distance.
Prognastic calculation of the time of pollutant reaching the groundwater table, based on migration parameters
Using the solutions proposed, alongside the data of experiments under dynamic conditions, it is possible to quantify the pollution pattern in the depth of subsoil changing with time. Figure 2 presents an example of calculation of some elements' concentrations in the layer composed of loesslike loam, in 25 years of operation of a tailing dump. The following data have been introduced: thickness (L) of the aeration zone -2.5 m; rate of sewage filtration (v) - 0.03m/day; heavy metals concentration - 10 p.p.m.
As seen in Fig. 2, the subsoil layer covering the aquifer does not provide for groundwater pollution protection from cadmium and manganese during the given time period.
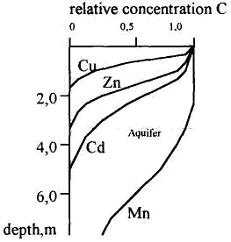 Heavy metals spread in subsoil layer after 25 years of tailing dump exploitation.
When solving the problem soft groundwater pollution control, the permissible time period of tailing dumps operation must be known. The permissible time period of operation (Tiim) is determined as the period of the whole retardation of all the pollutants within the screening layer.
The following equation may be used for permissible time calculation:
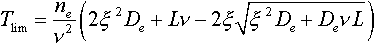
L - thickness of the aeration layer; 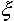 - is a parameter describing maximum permissible concentration of a pollutant at the lower boundary of the aeration zone.
CONCLUSIONS
A subsoil layer of the aeration zone in the foundation of waste disposal sites in many cases can be regarded as a reliable geochemical barrier for heavy metals.
The integrated approach to the assessment of subsoils as a screen, permits one on the basis of field and laboratory studies and theoretical calculations to forecast the process of pollutant migration in different subsoils and to determine the permissible time of soils functioning as geochemical barriers.
Migration parameters determination in laboratory experiments gives the possibility of calculating the necessary thickness of the man-made clay screen which will protect the aquifer from heavy metals during the designed life time.

|
|
|

