Лаборатория геологии техногенных процессов
Maximovich N.G., Menshikova E.A., Kazakevich S.V. Reseach of the possibility of increase of ground waters` agressiveness during the construction on pyritiferouns clay // International Symposium "Engineering geological problems of urban areas": Proceedings [Electronic resource]. Ekaterinburg, Russia 30 July - 2 August 2001. Ekaterinburg, 2001. - Electronic optical disks (CD - ROM).
Reseach of the possibility of increase of ground waters' aggressiveness during the construction on pyritiferous clay soils
Maximovich N.G., Menshikova E.A., Kazakevich S.V.
Abstract: Western part of Kurgan Region has been chosen to examine lithologic-mineral and geochemical peculiarities of Cheganskaya suite of Paleogene blue-gray clays containing pyrite. Results of the experiments on oxidizing pyrite with thiobacteria present in the clay have been presented. A possibility of ground waters' aggressiveness increase has been assessed when construction is held on pyritiferous clay soils. Proposals on protecting underground constructions from aggressive media have been considered.
Cheganskaya suite of Paleogene blue-gray clays is a foundation for the constructions planned in the western part of Kurgan Region. Clays, according to the geologic engineering investigations, is characterized by its aggressiveness to steel, its sulphate aggressiveness to concrete of normal permeability. The ground waters on the building sites are aggressive to concrete due to their total acidic and sulphate nature.
The mineral composition of clays of Cheganskaya suite, the presence of pyrite in particular (up to 0,8 %), determines the formation of its aggressiveness to a great extent. As practice shows, construction and utilization of buildings on such soils is accompanied with the increase in total acidic and sulphate aggressive nature of waters and soils. With this respect, a possibility of a change in chemical composition and aggressive characteristics of the ground waters and soils under construction has been assessed.
The clays of Cheganskaya suite was formed owing to the diagenetic transformations of prehistoric sea basin sediments. Marine conditions of the shelf zone existed on the territory of Zauralye from about the Turonian age of the Cretaceous period till the Early Oligocene /1/. The deposits of Cheganskaya suite constitute sediments of a rather shallow sea basin of lower saltiness, moderate temperature, alkalescent medium. Stagnant hydrodynamic conditions promoted a general pyritization of the sediments.
Cheganskaya suite sediments are regionally spread across the territory of Zauralye, except for the places eroded by water. The lithologic composition of the formation is homogenious over the huge area and is typically represented by greenish-gray clays of various tint from coarse- to thin-laminated, due to gouges and slate bands of micaceous- quarzous aleurite and fine-grained sand, while pebble and gravel are less often met. In the clay series can be met concretions and lenticles of siderite and phosphorite, mature nodular bands are rather rare to come across. The presence of small concretions of pyrite, crystals and growths of gypsum is typical of clays. According to its mineral composition, clays is referred to the montmorillonite group /1/.
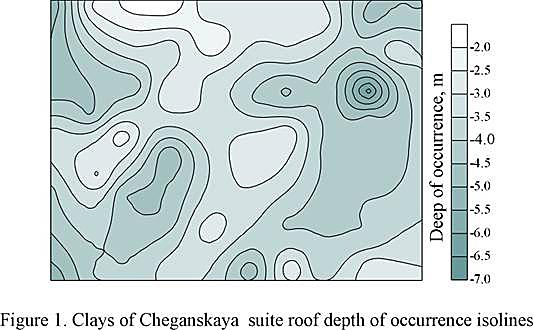
On the territory examined, according to drilling samples, Cheganskaya suite is represented by blue-green clays of foliaceous-bedded structure due to aleurite interlayers and fine-grained sand of quortzy-micaceous nature. The presence of average- and coarse-grained quortzy-micaceous sand interlayers with well-rounded pebble in them is less often observed. Sand and aleurite are, as a rule, of subordinate importance. There are gruss and rock waste made by marl, and organogenous limestone, as well as crystals and concretions of gypsum in the clays. The thickness of Cheganskaya suite on the territory examined, according to the research data, ranges from 27 to 39 m. The roof bedding depth on the building site, according to the drilling data, is within 1,5-6,8 m (fig. 1)
According to clays of Cheganskaya suite bulk sample X-ray spectrographic analysis data, its mineral content is made up of quartz, feldspar (albite and microcline). Clay minerals are represented by montmorillonite (up to 40 %), illite (up to 24 %), kaolinite (up to 18 %). Almost all samples have chlorite (1-18 %). Siderite (up to 17 %), gypsum (up to 15,3 %), jarosite (up to 6 %), pyrite (up to 0,8 %) are part of the clay make-up.
Clays of Cheganskaya suite aquatic extract is characterized by a well defined sulphate composition: it accounts for 0,7-4,8 g/kg of the total. Among cations prevail sodium and potassium (1,1 g/kg of the total), calcium accounts for 0,8 g/kg of the total. An overall amount of aquasoluble salts is considerable and constitutes 1,3-6,6 g/kg of the total. The dominant hydrogen index corresponds to alkalescent medium (pH 7-7,5). Clays of Cheganskaya suite aquatic extract in some places of the territory is characterized by hydrogen index low value (pH up to 4,3)
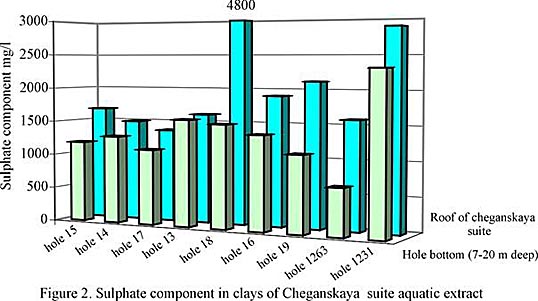
The analysis of change in sulphate-ion component in clays of Cheganskaya suite aquatic extract as it goes down, allows to note a general trend of its increase in the upper part of the formation. Here, as a rule, the sulphate- ion component goes up by 1,1-1,4 times (for some samples it is more than twice as much) than in the clays picked out from a hole bottom, within an interval of 7 - 20 m deep (fig. 2)
There are considerable amounts of aquasoluble trace elements' forms (mg/kg of the ground) in the aquatic extract: Mn (up to 38), Sr and Ni (up to 5), Zn (up to 4), Ba and Co (up to 3), Сu (up to 0,9), Li (up to 0,5).
The ground waters on the territory examined occur as deep as 0,0-1,85 m and are characterized by mainly hydrocarbonate-sulphonatrium and hydrocarbonate-natrium-sulphate composition. The mineralization generally varies from 0,5 to 1,2 g/l. The hydrogen index value ranges from 6,4 to 8,0. The content of macrocomponents varies within the range of (mg/l): HCO3- 128-702, SO42- 76-352, Cl- 4-234, Ca2+ 12-391, Mg2+ 12-431, Na++K+ 23-403. The nitrogen compound concentration doesn't exceed (mg/l): NO3- 4,8, NO2- 2,3, NH4+ 3,2. The ferrum component is no more than 0,1 g/l, aluminum is 0,6 mg/l.
A microcomponent composition of ground waters is high in manganese content (up to 4,4 mg/l). Other trace elements' content doesn't exceed (mg/l): Sr - 1,9, Ti - 0,7, Ва - 0,3, Ni - 0,2, Li and Ge, - 0,1, Ce - 0,09, Zn - 0,08, Cr - 0,04, La - 0,03, Со, V and Cu - 0,02, Y - 0,01, Nb - 0,008, Mo - 0,006, Sc, Pb and Ga - 0,002.
According to the lithologic-mineralogical investigation carried out all over the examined interval, the clays has pyrite concretions, which earlier, according to the engineering geological research materials, had been defined as flinty. Pyrite concretions are mostly of rounded flattened shape, less often they are of irregular angular, foliaceous shape. The biggest samples are as big as 5 cm in diameter and weigh as much as 70 g (fig. 3). The formations are confined to dark gray aleurite interlayers, the color of which is conditioned by pyrite of globulite structure. Clayey fraction elutriation results of some core samples of Cheganskaya suite testify to the fact that pyrite concretions make up 3 g/kg of the soils.

The analysis of lithologic mineral and geochemical peculiarities of Cheganskaya suite clay allows to speak about a process of pyrite oxidation at present taking place in some localities. Indications of oxidation are:
- sulphate-ions increase in the formations roof;
- aquatic extract medium acid reaction;
- a set of minerals' presence in clays, typical of sulphur - acidic process: gypsum, jarosite, siderite;
- presence in clays of thiobacteria at places with low value of hydrogen in aquatic extract medium.
Thiobacteria are an important factor of a sulphur- acidic process. Representatives of Thiobacillus play a key part in oxidation of a wide range of sulfur compounds and sulphate. Having powerful fermentation qualities, thiobacteria can compete in oxidizing activity with chemical oxidation of sulphide metals, elemental sulphur, bivalent metal sulphate. The speed of bacterial oxidation of bivalent metal sulphate in an acid medium is known to be hundred thousands -millions times as fast as that of chemical oxidation.
Thiobacillus ferrooxidans are the most important type to do with pyrite oxidation /2/. This type development optimum is at the pH value of 1,8-3,5, and its existence limits are within 1,5-4,8. Being underactive, they can be met in natural conditions both in neutral and alkalescent waters /2/. In ore deposits' underground waters thiobacteria are widely spread and active not only in the waters of oxidation zone, but in much greater depths (up to 400m) /3/. As a rule, Т. ferrooxidans are met in associations with other representatives of this kind, Т.thiooxidans are most frequent among them.
At different stages of pyrite's oxidation, a complex series of various compounds occur, sulphuric acid, sulphate of bivalent and trivalent metal being the main. As the oxidation goes on, the medium gets saturated with sulphuric acid, and the sulphates grow in content. Oxidation products affecting the surrounding rocks and minerals, cause formation of new mineral types.
In the mineral complex of sulphur-acidic process, a significant part is played by sulphate minerals. Most of the sulphate minerals of metal are extremely unstable. Minerals of jarosite group and jarosite itself are the most stable among the metal sulphates. This mineral is fixed in water and in relatively weak solutions. It is formed as a result of the many various reactions of sulphur acid and ferruginous sulphates with various minerals within the oxidation zone, illite in particular according to the data /4/. Т.ferrooxidans bacteria play impotent role in the formation of jarosites in nature according to the data published /5/.
The interaction of sulphate and sulphuric acid with carbonate rocks, limestone and dolomite primarily, creates favorable conditions for sedimentation of sulphates because of a sharp decrease in the acidity of medium. On meeting with carbonates of acid sulphate solutions, the neutralization of sulphur acid takes place first of all. Gypsum in the clays of Cheganskaya suite (aquatic sulphate of calcium) and siderite (carbonate of ferrum) prove to be a result of such interaction.
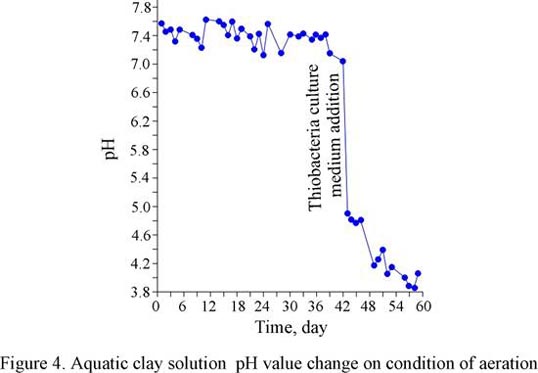
An experiment on oxidation processes has been fulfilled to study causes of clays of Cheganskaya suite aggressiveness origin. Given the fact that a biological factor plays a considerable part in a pyrite oxidation process, some thiobacteria have been developed from a portion of real existing clays of Cheganskaya suite. To do this, the culture medium for thiobacteria and Thiobacillus ferrooxidans, some samples of blue-green clays of Cheganskaya suite were brought in, characterized by the acid medium of their aquatic extract. A thin film as well as its turbidity, decrease in the hydrogen value, and presence of sulphates testified to the fact that the bacteria developed well enough in the culture medium.
According to the research conducted, blue-green clays in hydro-aerial conditions coupled with bacteria activities increase the acidity of water medium contacting them; they also act as a source of sulphate-ions getting into the solution. Thus, some samples of aquatic-clayey solutions within 30 days showed the hydrogen value going down which equaled 1 (it corresponds to a tenfold increase in sulphate-ion content) (fig. 4).
Construction activities accompanied with excavating pits and piling up the soils in the upper parts as well as drainage of the series will promote the increase in oxidation processes as oxygen will be able to easily access the rocks. The development of the process of pyrite oxidation will bring about a decrease in the medium hydrogen value, and sulphate entering into the ground waters, which in its turn will lead to an increase in sulphate and general acidic aggressiveness of the soils and ground waters.
Taking into account the processes mentioned above and the responsibility of a construction, soils and ground waters (according to CNaR 2.03.11-85 /6/) should be referred to as harshly aggressive to concrete of normal perversity on portland cement because of the sulphate content exceeding the norm and their general acidic aggressiveness. To make a rough forecast on changes in aggressiveness and chemical composition of ground waters, it is possible to use values mentioned in the areas where the considered geochemical processes are actively taking place at present.
Given the high costs of protecting underground constructions from harshly aggressive media, it could be advisable to erect geochemical barriers /7/ as a possible means of lowering the aggressiveness of soils and ground waters caused by pyrite oxidation. Neutralization of oxidation products could be reached by adding limestone into the soils when construction pits are filled up again.
References
- Umova L.A., Tsaur G.I., Shatrov V.P. Paleogeography of the Eastern Slope of the Urals and Zauralye during the Cretaceous and the Paleocene. -Sverdlovsk, 1968 (Russian).
- Kramarenko L.E. Geochemical and search meaning of microorganisms in the ground waters. - L.: Nedra, 1983 (Russian).
- Kramarenko L.E. Mineral deposit underground waters bacterial biocoenosis and their geological significance/Microbiology, 1962. - Vol.XXXI. -Edit.4 -694-701 (Russian).
- Hawkins A.B., Pinches G.M. Cause and significance of heave at Llandough hospital, cardiff Case history of ground floor heave to gypsum growth // Quarterly Journal of Engineering Geology. - London, 1987. - Vol. 20. - P.41-57.
- Tchukhrov F.V., Lyalikova N.N., Gorshkov A.I.. On Role of microorganinsms in jarosite formation.//Rep. Sc.Acad. USSR, 1978. Vol.241. -#4. -p.p. 929-932 (Russian).
- Construction Norms and Rules (CNaR) 2.03.11-85 Protection of constructions from corrosion. Gosstroi USSR. M.: Stroiizdat, 1985 (Russian).
- Мaximovich N.G., Blinov S.M. The use of geochemical methods for neutralization of surroundings aggressive to underground structures // Proceeding 7 Int. Congress Ass. of Engineering Geology.-V.5.-Portugal, Lisboa,1994.-P.3159-3164.

|
|
|

